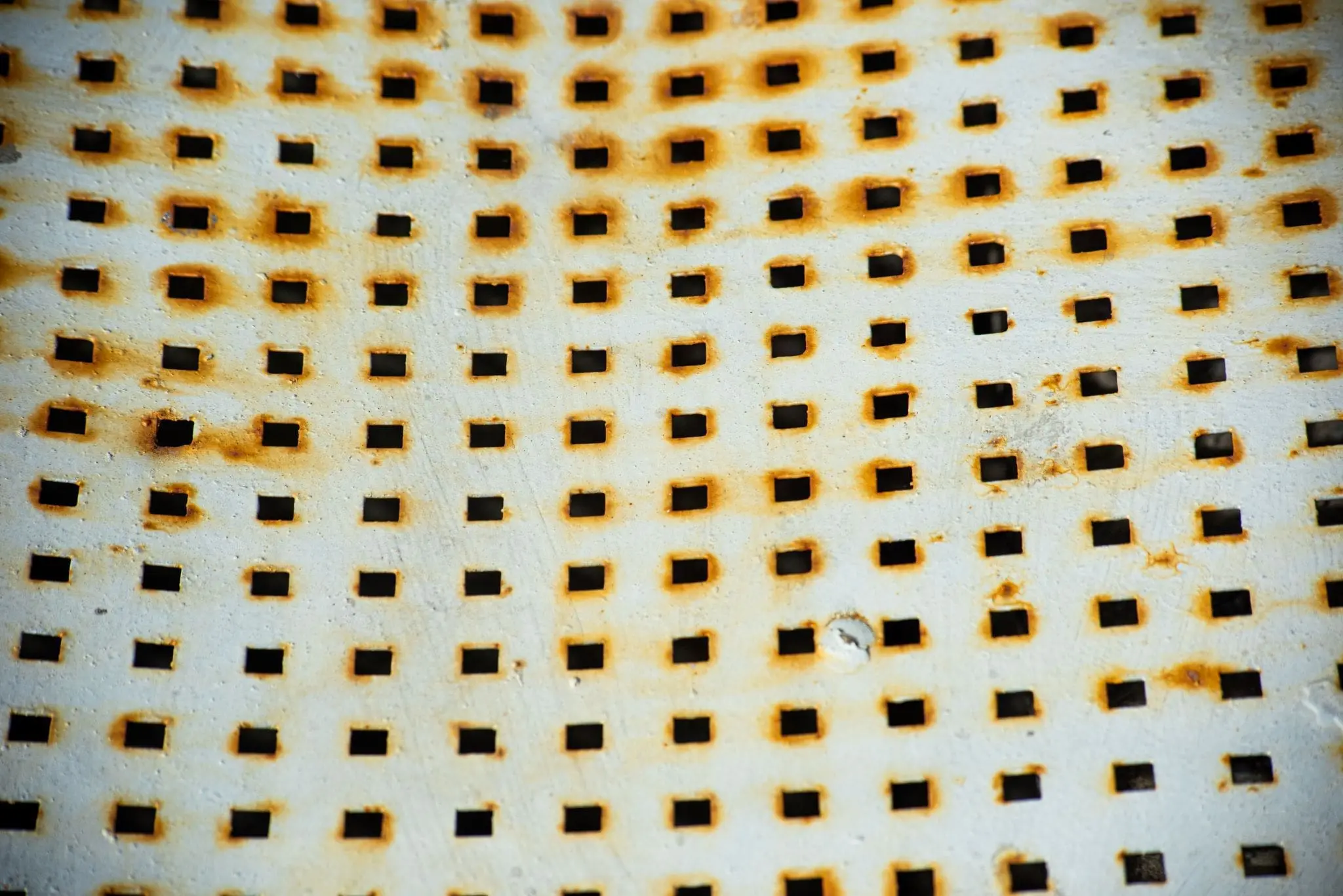
Pitting corrosion is undoubtedly the most dangerous type of metal erosion. Some experts believe it was responsible for the catastrophic collapse of the U.S. Highway 35 bridge between Point Pleasant and Kanauga over the River Ohio that killed 46 people in 1967. A small crack in one of the I-beams was thought to have been the result of metal fatigue induced by pitting corrosion.
Within chemical processing plants, corrosion in metals and metal alloys can also cause major failures. Cracking due to corrosion in the walls of a pressure vessel resulted in an explosion at a crystal manufacturing company, a fire occurred when severe corrosion resulted in titanium pieces coming in contact with steam and self-igniting, and fires and chemical leaks due to corroded valves and piping are all examples of these types of failures.
Heat recovery steam generators, steam turbines, mixing tanks, heat exchangers, storage tanks, and transfer pipelines are all pieces of chemical processing equipment subject to corrosion and potential failure. Low pH vapors present in these facilities are by definition acidic and can penetrate organic coating materials affecting the metal behind them, allowing for various types of corrosion, including pitting.
Understanding how corrosion occurs and how to repair and prevent it can save companies and business owners time and money while ensuring the safety of their team members and surrounding communities.
This post explores the effects of pitting corrosion in more detail. Follow ColdSpray.com to learn more about how cold spray technology can repair and restore metal surfaces affected by corrosion and apply corrosion-resistant coatings to prevent further damage.
How Does Pitting Corrosion Occur?
Pitting corrosion is a common problem anywhere metal components and structures are employed. Many metals protect themselves against corrosion by forming a thin “passive film” on the surface. For example, aluminum quickly oxidizes on the surface. That oxide layer then shields the bulk aluminum from further oxidation.
Other metals undergo similar reactions. If that film is disrupted (by scratching) or if it has a weak spot (such as an inclusion or a crystalline defect), then that small area becomes “depassivated”. Corrosive agents can now reach the depassivated area, which may have a different electrochemical behavior than the remaining (and usually quite large) passivated area.
This promotes ongoing corrosion. Tiny pits, too small to see, form on the surface, rapidly penetrating the metal while leaving the surface apparently free of corrosion. It attacks metals and alloys such as aluminum, steel, iron, copper, and chromium.
One of its most challenging characteristics is that it’s difficult to detect. At first glance, it is easy to overlook based on surface damage alone. However, it can cause devastating damage to metal structures within a short period if left unidentified.
Pitting corrosion is more aggressive when metal structures are exposed to chemicals with hypochlorite, bromide, and chloride ions. While fluoride and iodide seem to inflict less harm, sulfides speed up pitting corrosion. They also make metals more susceptible to this type of corrosion.
Exposing metals to thiosulphates is equally dangerous because it leads to electrochemical reduction. During this reaction, sulphidation occurs on the metal surface. The oxidizing cation quickly results in metal perforation. Although pitting corrosion may occur without oxygen, chlorides are much more dangerous in the presence of oxygen or hydrogen peroxide.
Types of Pitting Corrosion
Classification of pitting corrosion hinges on the shape, size, penetration pattern, and direction of pit formation. Here are the types of pitting corrosion:
- Sub-surface
- Shallow-wide
- Undercutting
- Elliptical
- Horizontal grain attack
- Vertical grain attack
Does Pitting Corrosion Attack Coated Metals?
Even though some metals may have a protective film or coating, they are still at risk of pitting corrosion. Consequently, chromium, cobalt, aluminum, copper, passive iron, stainless steel, and other alloys are susceptible to pitting corrosion.
In such scenarios, seemingly minor damage to the coating invites a series of chemical reactions that result in the formation of pits. Within a short period, pitting corrosion spreads into the larger surface area of the metal. Since this happens inwardly, the protective film becomes ineffective. The erosion becomes widespread and compromises the integrity of the metal.
How Does Pitting Corrosion Compromise Metal Assets?
What makes pitting corrosion destructive is that it damages metal assets inwardly. On the surface, it manifests itself as a small, hard-to-detect perforation. However, most destruction occurs inside the metal, leading to material weight loss. This weight loss leads to metal fatigue resulting in the metal experiencing stress fractures and developing cracks. Over time, the metal structure becomes too weak and fails.
For example, when a beam of metal that supports a racking system loses its structural integrity due to pitting corrosion, it can no longer support the weight of the stock but goes unnoticed. Or at least it goes unnoticed until the rack collapses, potentially resulting in injuries to staff and damage to inventory and equipment. A more dramatic and unpleasant example would be a pressurized tank full of hot, dangerous chemicals suddenly giving way.
Common Causes of Pitting Corrosion
In most cases, pitting corrosion will not just occur without some form of exposure to chemical and oxidation agents. Part of the reason it attacks coated metals is that there are weak spots or design flaws. Here are some causes of pitting erosion on metal assets:
- Cracks in the protective film
- Unevenness in metal thickness
- Abrasions on metal surfaces
- Aggressive fluid flow on metal surfaces
- Inadequate oxygen
- Stagnant moisture in the environment
- Inadequate alloying elements on metal assets
- Crystal defects, inclusions, and other disruptions.
Preventing Pitting Corrosion
There are several ways to prevent pitting corrosion. The first one is choosing the right type of metal for the application and environment. With a sound understanding of how various metals react with the environment, it is easy to specify the most suitable material for the job. For instance, higher alloy metals have a greater resistance to pitting corrosion than their counterparts.
Second, controlling the environment, where possible, also helps keep pitting corrosion at bay. When metal structures are indoors, environmental factors such as the pH, chloride concentration, and temperature increase the chance of corrosion. Mitigating these circumstances can go a long way toward preventing the need for component and structural repair or replacement.
Additionally, the application of various protective films assists in protecting metal assets from pitting corrosion. However, there is a caveat. Slight damage to the coating from an abrasive material or an acidic environment can cause a break in the film, exposing the metal to chemicals or oxidation agents. When this happens, the asset will once again be susceptible to corrosion.
Adding highly corrosion-resistant coatings to metal components and structures is another avenue for pitting corrosion prevention. Metals such as aluminum, commercially pure titanium, pure nickel, and nickel-based superalloys can be added through the supersonic particle deposition process, also known as cold spray. This, provides excellent corrosion resistance. You can also apply hard ceramic/metal composite powders to reduce sliding wear and erosion caused by abrasives that may be present in the environment.
How to Fix Pitted Metal Surfaces
When pitting corrosion has already occurred, there are a few ways to fix the problem. A simple but not necessarily durable solution is to clean the surface metal with wire brushes or abrasive blasting techniques and apply a new corrosion-resistant film layer.
If pitting corrosion has not yet compromised the piece’s structural integrity, high-pressure cold spray (HPCS) can also rapidly apply nickel, bronze, and many other metals to restore dimensional features that are now out of tolerance due to surface corrosion and wear. In the process, these coatings can also prevent surface crack formation and growth by strongly bonding to the substrate and imparting compressive residual stresses, actually enhancing the base metal strength.
Follow all the latest developments in cold spray technology, equipment, and resources, by checking out ColdSpray.com and requesting more info.